Abstract
Background: Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a clinically aggressive myeloid malignancy that has a poor prognosis. BPDCN occurring concurrently with other hematologic malignancies has been observed in approximately 10-20% of patients (pts) with BPDCN having prior or concomitant hematologic malignancies (PCHM); myelodysplastic syndrome (MDS) and chronic myelomonocytic leukemia (CMML) are the most common. Such pts likely have a unique disease biology, which may impact the response to therapy. BPDCN derives from plasmacytoid dendritic cells that overexpress CD123 (also known as interleukin-3 receptor alpha). Tagraxofusp (TAG), a first-in-class CD123-targeted therapy, is the only FDA- and EMA-approved treatment for pts with BPDCN. The pivotal trial (NCT02113982) demonstrated that TAG 12 mcg/kg resulted in high (overall response rate [ORR] = 75%) and durable (median duration = 25 months) responses after 3 years of follow up (Pemmaraju et al. JCO 2022 doi: 10.1200/JCO.22.00034) in first-line (1L) pts; the safety profile was well characterized and manageable. Herein, we report a subgroup analysis of the pivotal trial that evaluates the safety and efficacy of TAG in 1L pts who had PCHM.
Methods: This was a multicenter, 4-stage, single-arm phase 1/2 trial that enrolled 1L (n = 69) pts with BPDCN. Pts received TAG intravenous infusions once daily on days 1-5 of a 21-day cycle at 7 or 12 mcg/kg in Stage 1 (dose escalation) and 12 mcg/kg in Stages 2 (dose expansion), 3 (pivotal confirmatory), and 4 (continued access). The main endpoints were complete response (CR; defined as CR + clinical CR [CRc; CR with residual skin abnormality not indicative of active disease]), ORR, overall survival (OS), and safety.
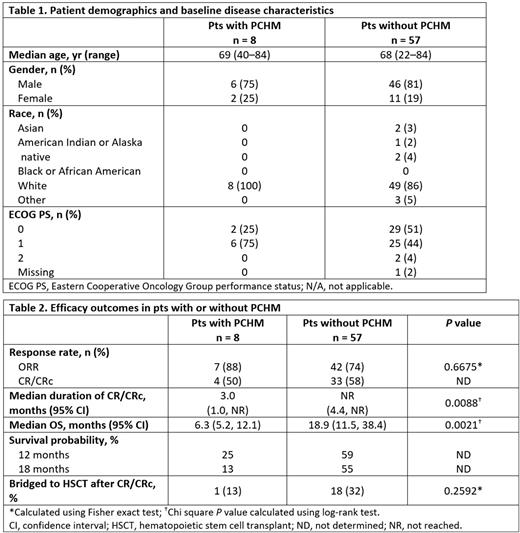
Results: In total, 65 1L pts received TAG at 12 mcg/kg. Of these, 8 (12%) had PCHM, one of whom had 2 different PCHMs; there were 4 pts with MDS, 1 pt with polycythemia vera, 1 pt with CMML, 1 pt with plasma cell myeloma, 1 pt with Hodgkin's disease, and 1 pt with lymphoma. Baseline characteristics were similar between pts with or without PCHM; respectively, median age was 69 years and 68 years, most pts were male (75% vs 81%), and the majority had ECOG PS 0/1 (100% vs 95%) (Table 1). Past medical history showed that the length of time pts had a PCHM prior to the BPDCN diagnosis varied, ranging from 4-14 years. The main efficacy outcomes are presented in Table 2. Similar rates of CR/CRc and ORR were seen across pts with and without PCHM. The most frequent (≥25%) treatment-related adverse events (TRAEs) for pts with PCHM were: increased aspartate aminotransferase (AST; 38%), increased alanine aminotransferase (ALT), weight gain, and hypoalbuminemia (25% each). No pts with PCHM experienced capillary leak syndrome (CLS). For pts with no PCHM, TRAEs occurring with an incidence ≥25% were increased ALT (56%), increased AST (53%), hypoalbuminemia (40%), thrombocytopenia (35%), pyrexia (32%), increased weight (28%), and nausea (25%); 12 (21%) pts had CLS.
Conclusion: While the numbers of pts are low, there is evidence that TAG has efficacy in 1L pts with BPDCN who had PCHM. High rates of response, similar to those reported in pts with no PCHM, were observed. TAG did enable 1 of the 8 pts with PCHM to be bridged to SCT. The safety profile was manageable and predictable. PCHM did not appear to predispose pts to different TRAEs. These data also highlight how BPDCN can frequently co-occur with other hematologic malignancies. This reinforces the importance of making the correct diagnosis of BPDCN to enable treatment with disease-directed therapy, and of monitoring for cytopenia, including thrombocytopenia, in pts with BPDCN vs other hematologic malignancies, as well as the need for longer follow up.
Disclosures
Pemmaraju:stemline: Consultancy; abbvie: Consultancy; immunogen: Consultancy; mustangbio: Research Funding; incyte: Consultancy; novartis: Research Funding; pacylex: Consultancy, Research Funding; samus: Research Funding; daiichi sankyo: Research Funding; cellectis: Research Funding; cellularity: Research Funding. Konopleva:Genentech: Consultancy, Other: grant support, Research Funding; Kisoji: Consultancy, Honoraria; Reata Pharmaceuticals: Current equity holder in private company, Patents & Royalties; Novartis: Patents & Royalties, Research Funding; Sanofi: Other: grant support, Research Funding; Rafael Pharmaceutical: Other: grant support, Research Funding; AstraZeneca: Other: grant support, Research Funding; Ascentage: Other: grant support, Research Funding; Agios: Other: grant support, Research Funding; Ablynx: Other: Grant support, Research Funding; Calithera: Other: Grant Support, Research Funding; Cellectis: Consultancy, Other: Grant support, Research Funding; Eli Lilly: Consultancy, Patents & Royalties, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Forty-Seven: Consultancy, Honoraria, Other: Grant support; Amgen: Consultancy; Stemline Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; F. Hoffman La Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Grant support, Research Funding; AbbVie: Consultancy, Other: grant support, Research Funding. Sweet:Gilead Sciences, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AROG: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; Syntrix Pharmaceuticals: Research Funding; berGenBio: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Curis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Mablytics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Stein:Amgen: Speakers Bureau. Vasu:Boehringer Ingelheim: Other: Travel support; Seattle Genetics: Other: Travel support. Rizzieri:Bayer: Consultancy, Honoraria; Celgene, Jazz, Gilead, Incyte, Amgen, Kite, AROG, Pharmacyclics, Seattle Genetics.: Honoraria; Mustang: Consultancy, Honoraria; Stemline: Consultancy, Honoraria, Research Funding, Speakers Bureau; Celltron: Consultancy, Honoraria. Wang:Pfizer: Consultancy, Honoraria, Other: Advisory Board, Speakers Bureau; Gilead: Consultancy, Honoraria, Other: Advisory Board; Stemline Therapeutics: Consultancy, Honoraria, Other: Advisory Board, Speakers Bureau; Abbvie: Consultancy, Honoraria, Other: member of data monitoring committee ; Daiichi Sankyo: Consultancy, Honoraria, Other: Advisory Board; Takeda: Consultancy, Honoraria, Other: Advisory Board; Macrogenics: Consultancy; Astellas: Consultancy, Honoraria; BMS/Celgene: Membership on an entity's Board of Directors or advisory committees; Dava Oncology: Consultancy, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Honoraria, Other: Advisory Board; Kura Oncology: Consultancy, Honoraria, Other: Advisory Board, Steering Committee, Speakers Bureau; Rafael Pharmaceuticals: Other: Data Safety Monitoring Committee; Genentech: Consultancy; Mana Therapeutics: Consultancy, Honoraria; Kite Pharmaceuticals: Consultancy, Honoraria, Other: Advisory Board; Novartis: Consultancy, Honoraria, Other: Advisory Board; GlaxoSmithKline: Consultancy, Honoraria, Other: Advisory Board; PTC Therapeutics: Consultancy, Honoraria, Other: Advisory Board. Kantarjian:AbbVie: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; ImmunoGen: Research Funding; NOVA Research: Honoraria; Pfizer: Honoraria, Research Funding; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Consultancy, Research Funding; Amgen: Honoraria, Research Funding; Jazz Pharmaceuticals: Research Funding; Takeda: Honoraria. Brooks:Stemline Therapeutics: Current Employment. Paley:Stemline Therapeutics: Current Employment. Mughal:Stemline Therapeutics: Current Employment, Current holder of stock options in a privately-held company; Oxford University Press: Other: financial benefit and/or patents; Informa: Other: financial benefit and/or patents. Lane:N-of-One: Consultancy; Qiagen: Consultancy, Honoraria; AbbVie: Research Funding; Stemline Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal